What is an Element (in terms of Chemistry)
?
Matter can be classified in various ways according to
its structure, behaviours and physical
and chemical properties.
The main classifications of matter include the
categories element
and compound,
either of which may also be called a "substance" (which
is a less specific term), and mixture
- of which there are also many sub-categories.
Definition of a Chemical Element:
An element is a chemical substance that
cannot be broken-down into any simpler substances by
chemical reactions. It consists of only one
type of atom,
though the atoms of an element may, or may not, join
together to form molecules
(that depends on the particular element and
so the structure of its atoms).
All
elements are included in the Periodic
Table.
List of facts about Chemical
Elements:
- Elements
consist of only one type of
atom - which may, or may not be joined
together to form molecules or large structures, so ...
- Elements
can exist either as atoms (e.g. argon)
or as molecules (e.g., nitrogen)
- Elements
cannot be broken down into a simpler type of matter by
either physical or chemical techniques - though
some larger elements break-down spontaneously due to
being radioactive.
- Elements
are listed in the periodic
table.
Symbols of Chemical Elements:
Every chemical element has its own symbol.
Examples of chemical symbols are N (for the element
nitrogen), He (for the element helium) and Pb (for the
element lead).
For more about symbols
of the chemical elements see pages listing
these:
Many elements are found in nature and so may be
called "naturally occurring elements". Other
elements have not been found in nature but can be
produced in the laboratory. A few more chemical elements
are thought to exist but are very rare and even if
produced would only exist for a very short time because
they are radioactive and would quickly decompose into
other elements whose atoms are smaller.
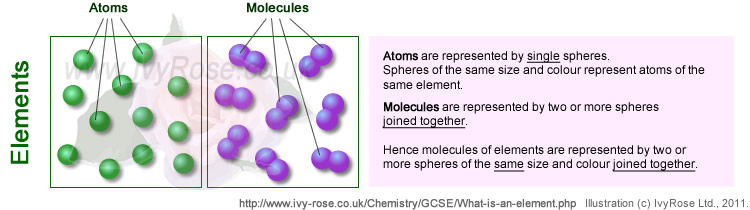
Do elements consist of atoms or molecules
?
Some elements exist in the form of atoms
e.g. the noble bases neon (Ne), argon (Ar), krypton
(Kr), xeon (Xe) and radon (Rn).
Other elements exist
in the form of molecules.
For example many common gases exist as diatomic
molecules e.g. oxygen (O2), hydrogen
(H2), and nitrogen (N2).
Note: This
is one of a series of simple pages introducing key
concepts in introductory chemistry. Other pages in this
section include elements,
mixtures and compounds and individual pages
about substances,
elements,
mixtures
and compounds,
plus pages about atoms,
molecules
and isotopes.
If you need further information ask
your chemistry tutor. |